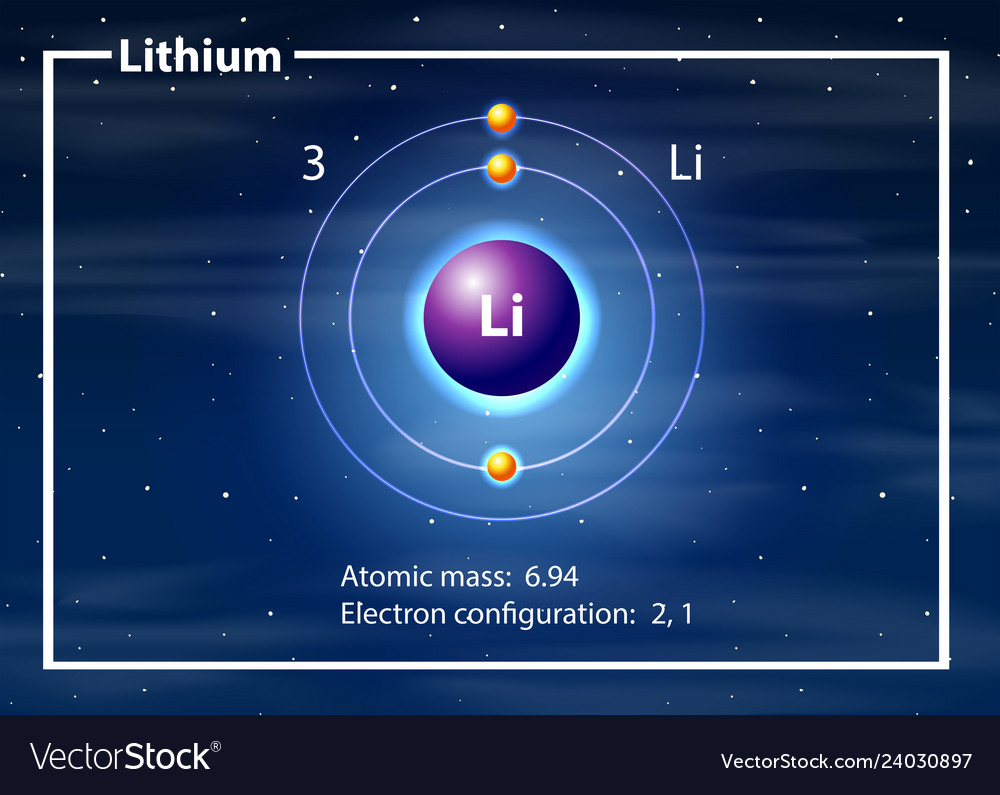
A lithium atom diagram Royalty Free Vector Image
Lithium metal is isolated electrolytically from a mixture of lithium chloride and potassium chloride . The nucleus of the lithium atom verges on instability, since the two stable lithium isotopes found in nature have among the lowest binding energies per nucleon of all stable nuclides.
Image Stylised Lithium Atom.png Elements Wiki
Lithium is the third element with a total of 3 electrons. In writing the electron configuration for lithium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the remaining electron for Li goes in the 2s orbital. Therefore the Li electron configuration will be 1s 2 2s 1.
Chemistry collection Imageshare
Lithium assumes a close-packed structure under ambient conditions and is a simple metal with a nearly free-electron-like band structure. Almost two decades ago, it was predicted using first-principles calculations that, somewhat counterintuitively, as the pressure increases and the average electron density rises, lithium undergoes a sequence of phase transitions in which the coordination.
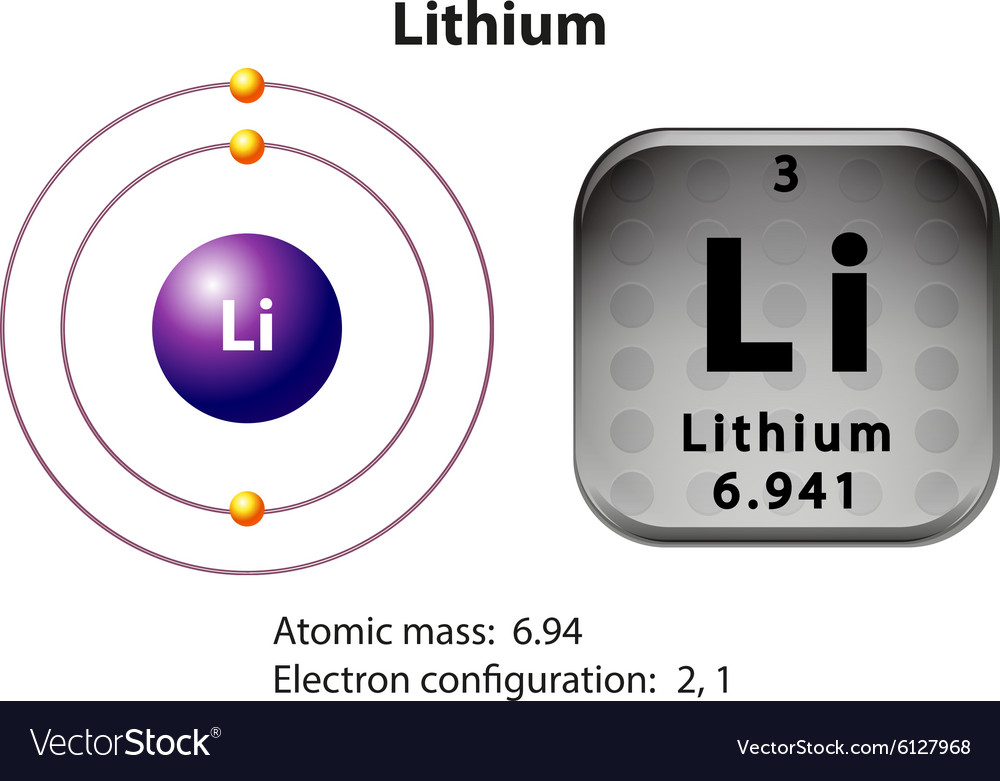
Periodic Table Lithium Electron Configuration Periodic Table Timeline
January 25, 2022 SCIENTIFIC ACHIEVEMENT Spectroscopy at the Advanced Light Source (ALS) and theoretical calculations at the Molecular Foundry revealed the intrinsic spectroscopic signature of lithium metal and explained the origin of previous contradictory reports. SIGNIFICANCE AND IMPACT
:max_bytes(150000):strip_icc()/lithiumatom-56a12c335f9b58b7d0bcc103.jpg)
What Is the Notation for Electron Configuration?
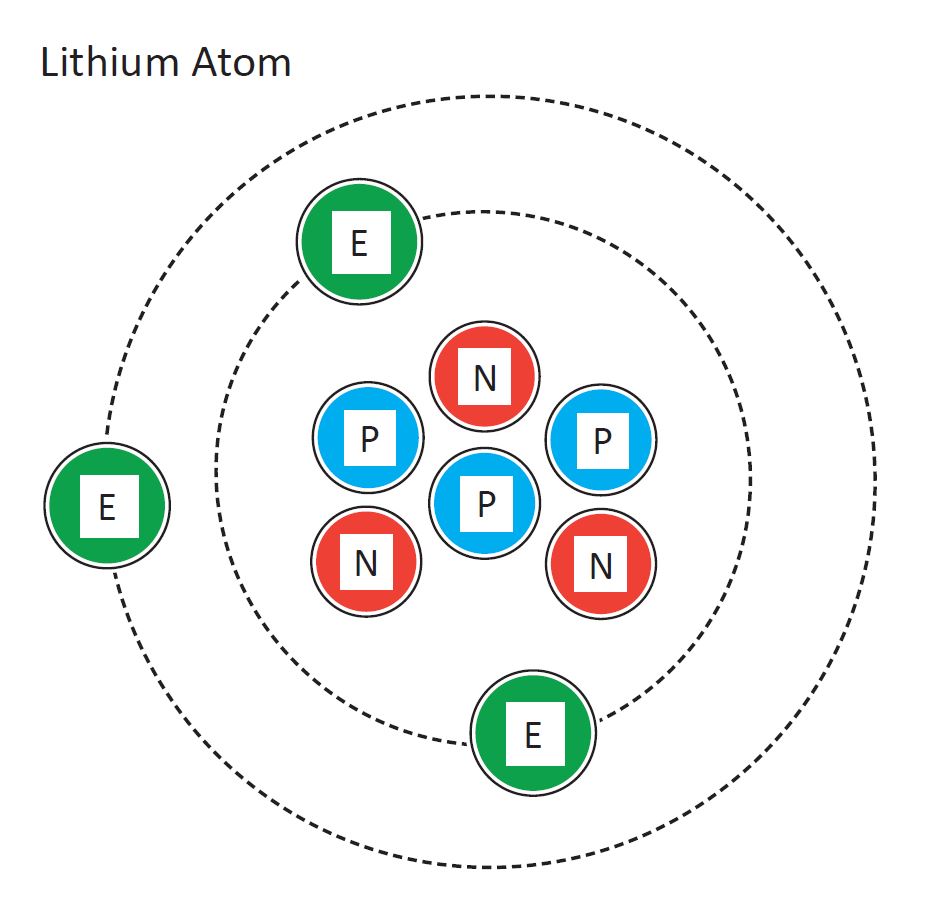
It should be clear from Plate 4 that when a lithium atom interacts with another atom, the 2s electron is far more likely to be involved than either of the two 1s electrons. In Lewis' terminology, it is a valence electron and occupies a valence shell. The pair of 1s electrons are a complete shell and form the kernel of the lithium atom. There.

Lithium, atomic structure Stock Image C018/3684 Science Photo Library
What is the electron configuration for lithium? The total number of electrons in lithium is three. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in lithium in specific rules in different orbits and orbitals is called the electron configuration of lithium.

3D Render Atom Struktur von Lithium isoliert auf weißem Hintergrund Protonen werden als rote
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the.

Help Me With Basic Chemistry How to Do Lewis Dot Structure (Simple)
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 6.25 ): The number of the principal quantum shell, n,

Lithium electron configuration Stock Image C029/5021 Science Photo Library
235 44K views 5 years ago For the Li+ structure use the periodic table to find the total number of valence electrons for Li. Once we know how many valence electrons there are in Lithium (Li) we.
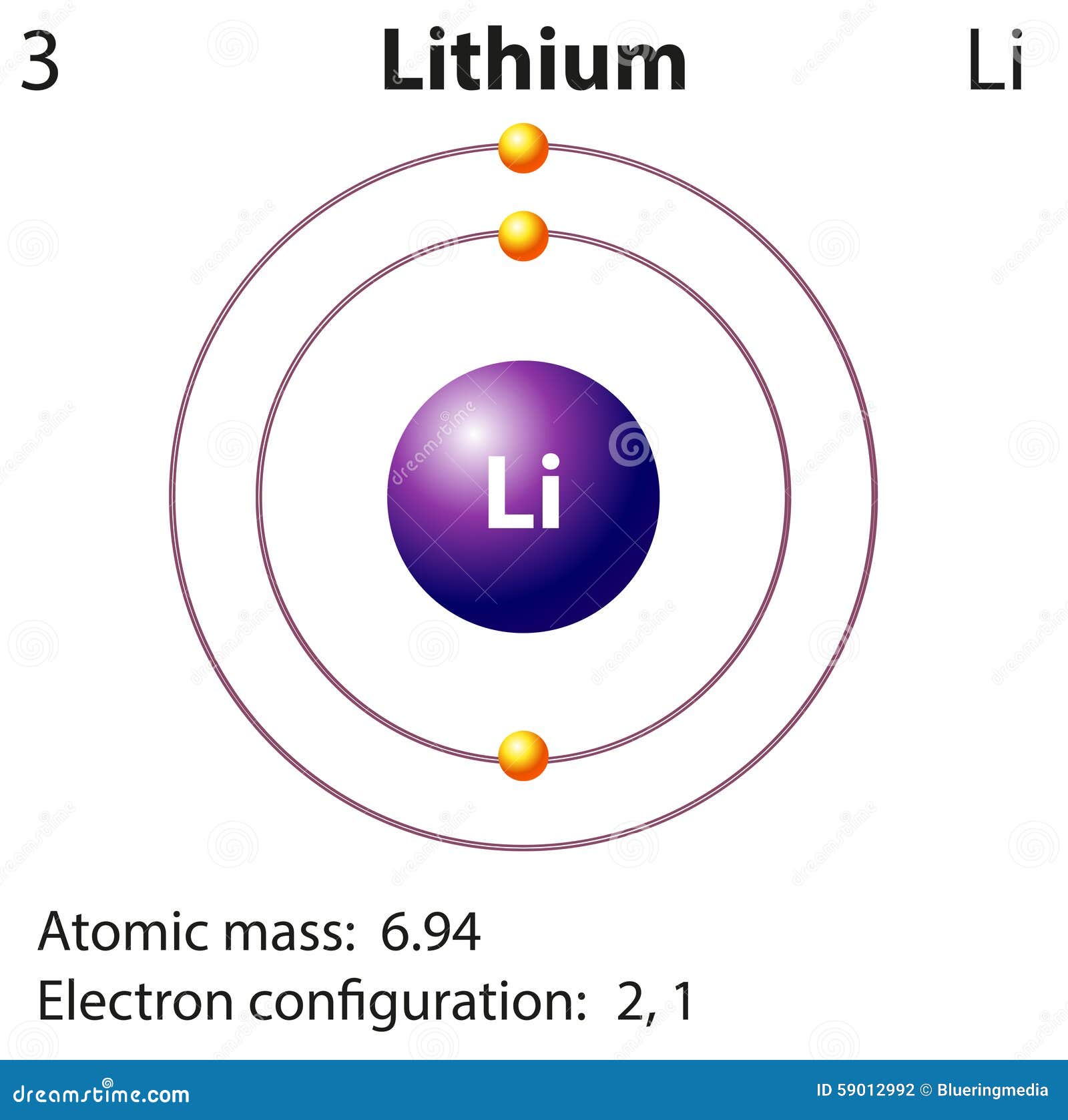
Diagram Representation Of The Element Lithium Stock Vector Image 59012992
Lithium-ion batteries (LiBs) are the leading energy storage technology for portable electronics and electric vehicles (EVs) 1, which could alleviate reliance on fossil fuels.
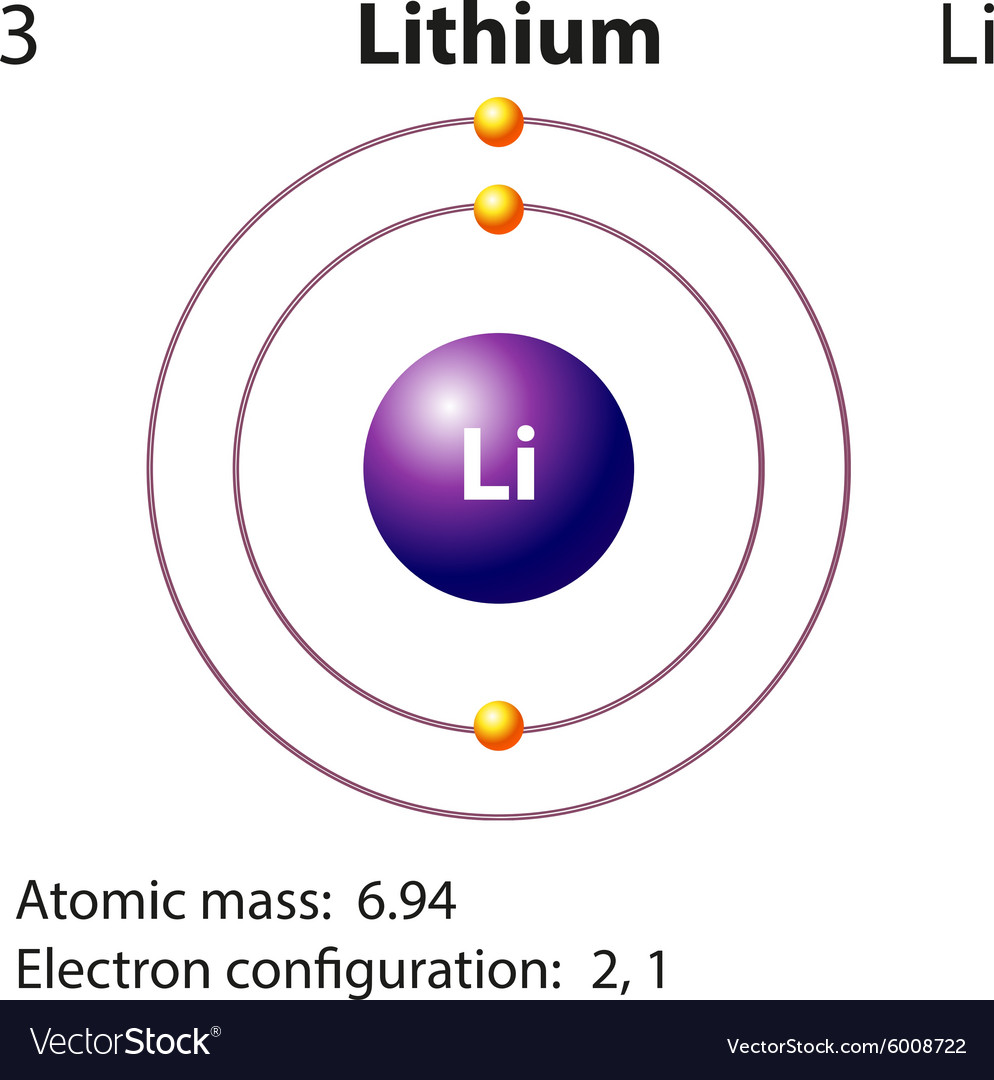
Diagram representation of the element lithium Vector Image
Introduction to electron configurations Google Classroom About Transcript Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Created by Sal Khan.
Facts About Lithium Properties and Uses Owlcation
Most technologically important electrode materials for lithium-ion batteries are essentially lithium ions plus a transition-metal oxide framework. However, their atomic and electronic.
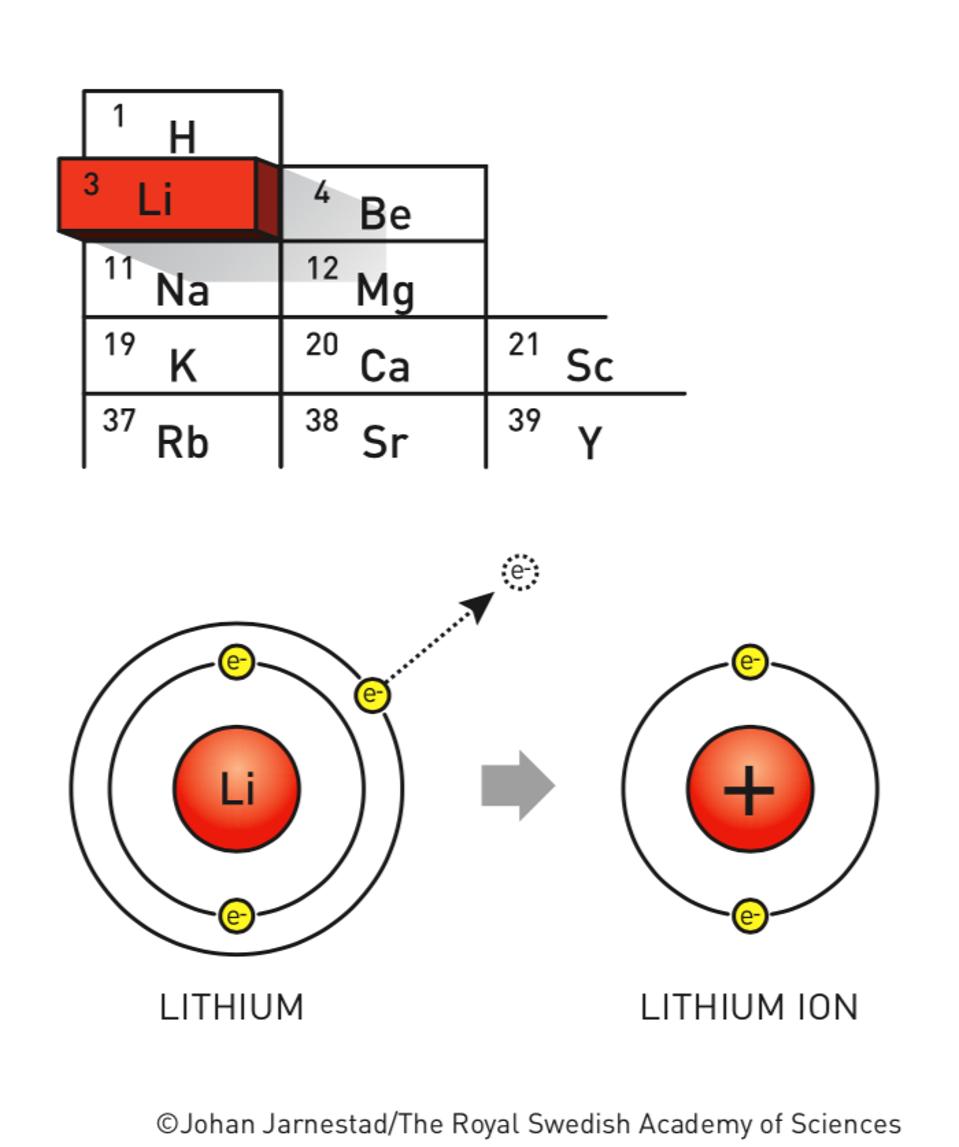
The Science Behind The LithiumIon Battery Research That Won 2019’s Nobel Prize In Chemistry
NEWS Revealing Lithium Metal's Electronic Structure January 25, 2022 This article has been adapted from this ALS science highlight. The last decade has seen extraordinary advancements in electron microscopy, enabling researchers to image materials at increasingly high resolutions.

Li+ Electron Configuration (Lithium Ion) YouTube
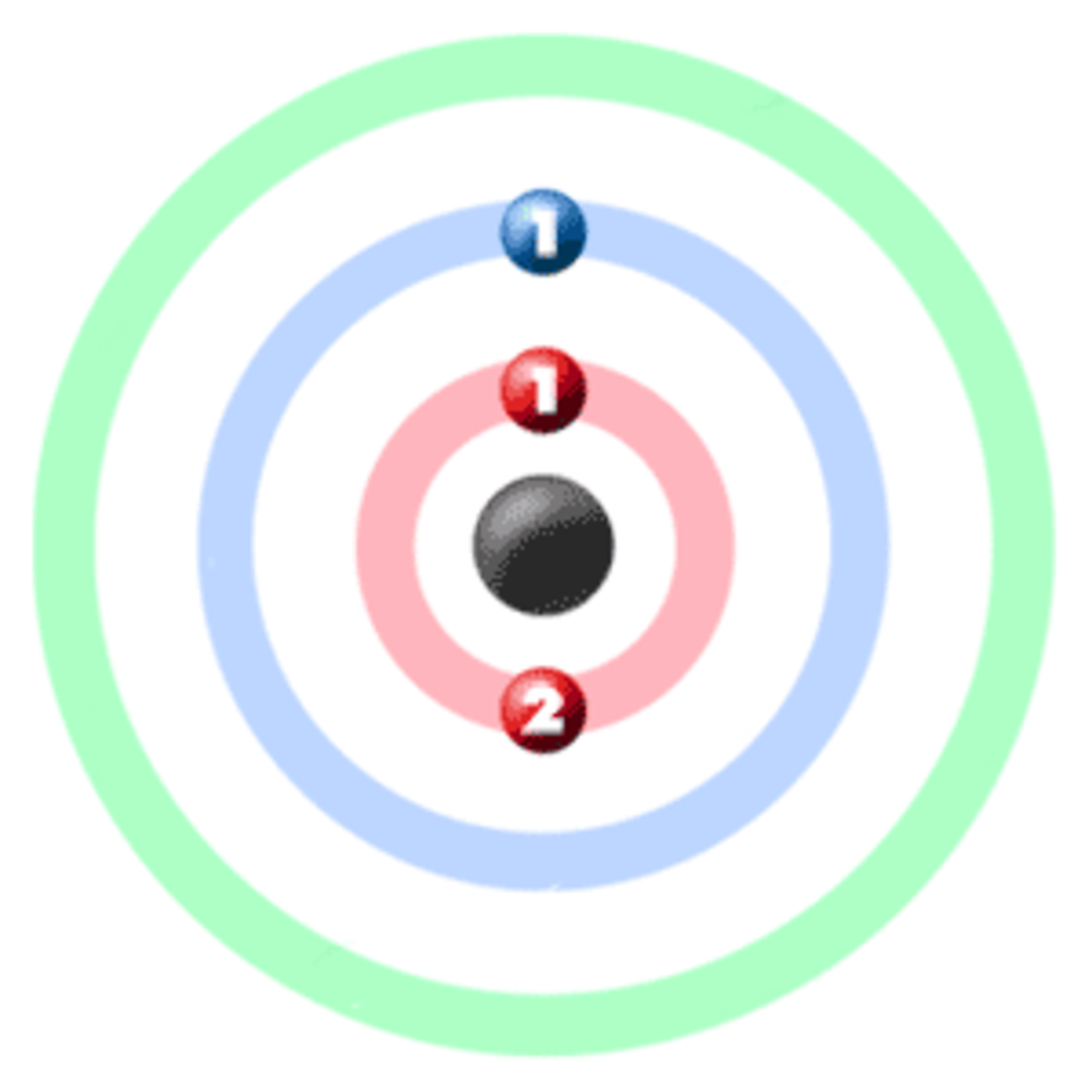
electrons are arranged. It can be shown as numbers or as a diagram. Electronic structure of lithium Take lithium for example. The diagram shows each shell as a circle around the , with each.
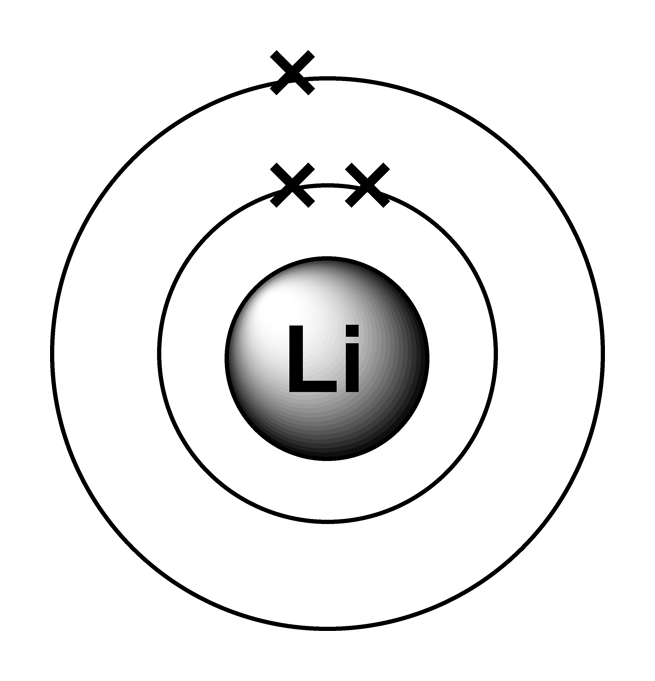
Electron arrangements
Help & legal Element Lithium (Li), Group 1, Atomic Number 3, s-block, Mass 6.94. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.
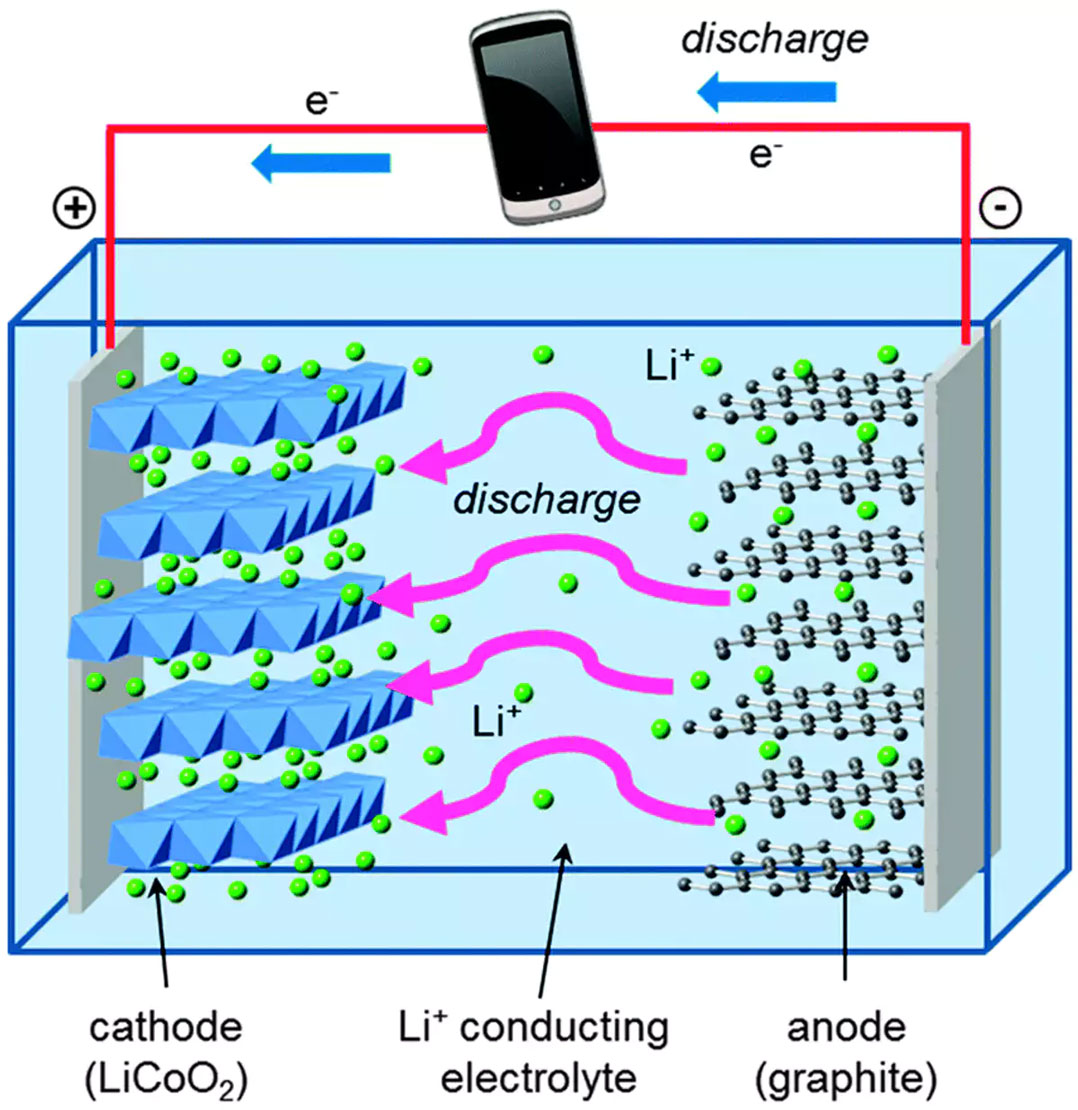
Explainer How lithiumion batteries work TechCentral
A step-by-step explanation of how to draw the Lewis dot structure for Li (Lithium). I show you where Lithium is on the periodic table and how to determine how many valence electrons it has..